
News
Home News Technical articles sterilizing filters should be as close to the point of fill as possible
Home News Technical articles sterilizing filters should be as close to the point of fill as possible
sterilizing filters should be as close to the point of fill as possible
2024-08-27 EternalwaterThis case comes from an experience of participating in the education of the International Pharmaceutical Engineering Management (IPEM) course. A student asked the teacher Lan a question. The general situation is as follows:
Here's my question: Which of the two images below is more in alignment with "sterilizing filters should be as close to the point of fill as possible "?
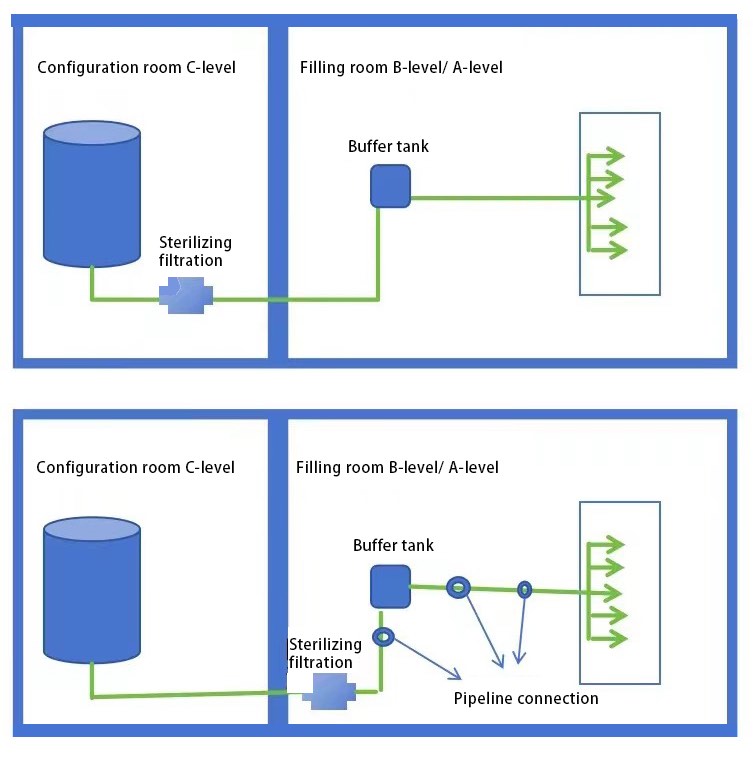
GMP Appendix 1 Article 75 Filtration and sterilization of non-final sterilized products shall meet the following requirements: (2) Measures shall be taken to reduce the risk of filtration and sterilization. It is advisable to install a second sterilized sterilizing filter to filter the liquid again, and the final sterilizing filter should be as close to the point of fill as possible.
Question: Is the proximity to the point of fill here physical proximity?
Let's take a look at Lan's explanation again. He said that "closed" does not mean physical proximity, but more refers to the need to minimize pipeline connections or use aseptic connections after sterilizing filtration. If it is an aseptic connection, a longer pipe can also be regarded as "closed". He believed that the inspector in the student's question may not be a native speaker of English, or may not have received enough training to correctly understand this sentence.
I personally agree with Lan's explanation, so the first one in the first image can reduce the aseptic risks more. When designing processes, companies should try to reduce pipeline connections or change them to aseptic connections, and do not only consider the proximity in distance.
Statement 1. The cases in this public account are all fictitious by the author, or have been fully reprocessed, and the values quoted are not actual values. If it still causes troubles to some companies, please contact us for deletion. 2. The articles published on this public account are all intended to promote exchanges and learning among colleagues in the pharmaceutical industry. Not for any commercial use.
Latest News
Read more
- Industry Application
- Life Sciences
- water treatment
- Industrial Filtration
- Food & Beverage
- Microelectronics
- Laboratory
- New energy battery
- Contact Us
- [email protected]
- +86-571-87022016
- +86-571-87293027
- +8613675899519
- Subscribe for Join Us!
- Join us and get detail information,technical parameter and new products etc.
CopyRight © Hangzhou Eternalwater Filtration Equipment Co., Ltd. 2002-2025
- [email protected]
- Jenny wu
- +8613675899519
- +86(571) 87022016

 EN
EN  ES
ES AR
AR JP
JP CN
CN




