
News
Home News Technical articles Application of sterilizing filtration products in eye drops production process
Home News Technical articles Application of sterilizing filtration products in eye drops production process
Application of sterilizing filtration products in eye drops production process
2024-03-06 Eternalwater Eye drops refer to sterile liquid preparations such as clear solutions or suspensions made from drugs and excipients for eye drops, used to prevent, treat or diagnose eye diseases. It is usually composed of main ingredients, pH adjuster, isotonicity adjuster, bacteriostatic agent and viscosity increasing agent. Commercially available eye drops are mostly aqueous solutions, with a few aqueous suspensions or oil solutions. Eye drops are currently the most widely used type of ophthalmic preparations, which can sterilize, reduce inflammation, dilate, miosis, and anesthetize the eyes."Chinese Pharmacopoeia" 2020 Edition General Chapter 0105 Ophthalmic Preparations describes " Eye drops refer to sterile liquid preparations made of raw materials and suitable excipients for instillation into the eyes ." In the production process of eye drops, water for injection needs to be mixed with drugs, and then the eye drops are made into finished products and filled into clear solutions, suspensions or emulsions; drugs can also be packaged in the form of powders, granules, etc. , prepare separate solvent and prepare a clear solution or suspension before use.
By type, eye drops are currently mainly divided into antibiotic eye drops, antiviral eye drops, antifungal drugs, glucocorticoids, non-steroidal anti-inflammatory drugs, cycloplegics, drugs to promote corneal repair, and anti-inflammatory drugs. Intraocular pressure eye drops.
In terms of function, eye drops are divided into two categories; one is health-care eye drops, which are mainly used to relieve visual fatigue and have health-care functions for the eyes. The other type is therapeutic eye drops, which mainly include eye drops for cataracts, glaucoma, dry eye disease and anti-inflammatory eye drops.
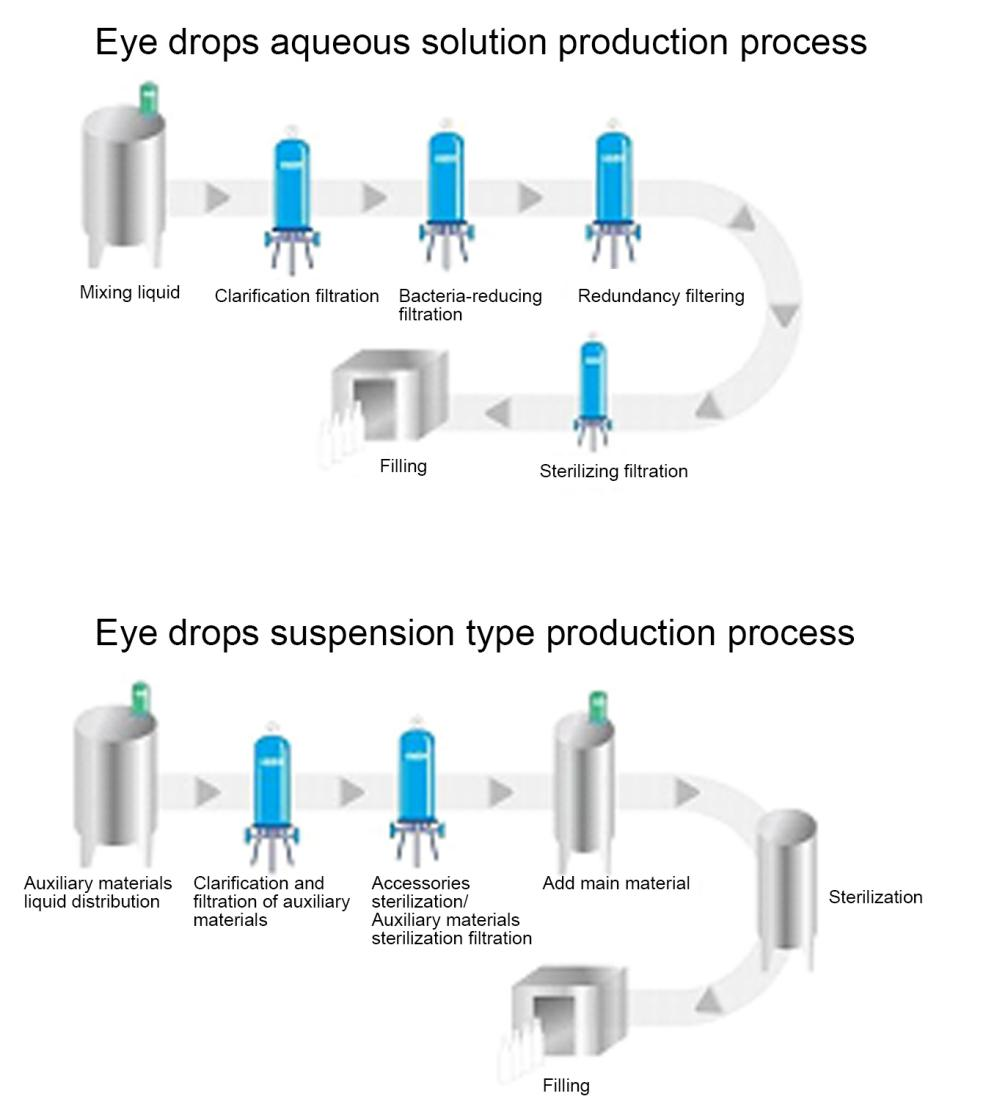
If there are no special requirements for the aseptic production process of eye drops, the process flow is generally required to consist of weighing of raw materials and excipients, preparation of medicinal solution, sterilization filtration , filling and other processes. Water for injection is generally used for preparation or dilution of medicinal solution. The sterile production process flow of common water-based eye drops in plastic bottles is shown in the figure below:
Eye drops are produced according to aseptic processes and the final product is a sterilized product, so the microbial quality of the finished product should be uniformly sterile. Therefore, the requirements for filtration are also extremely strict. Some eye drops have high viscosity and are very difficult to filter. Some eye drops contain very little preservatives and are easily adsorbed by conventional filters . Hengshui Company has a sound process verification experiment. The laboratory has complete management procedures, standard test procedures and standard operating specifications, and can conduct filterability experiments, adsorption verification, wetting integrity verification, chemical compatibility verification, and extractable content verification of eye drops. , verification of bacterial interception, etc., to protect customers throughout the process.
pleated filter cartridge factory
The General Principles of Ophthalmic Preparations in the 2010 edition of the Chinese Pharmacopoeia stipulates that eye drops are sterile preparations and requires that sterility items be inspected and comply with regulations. At the same time, the Sterile Drugs Volume of the "Drug GMP Guidelines" clearly includes the production of eye drops within the scope of sterile drug management, and emphasizes the use of aseptic processes to reduce potential contamination by particles, microorganisms and endotoxins.
Due to the constraints of the characteristics of sterile eye drops and the inner packaging materials in contact with the liquid, eye drops are generally non-terminal sterilized sterile preparations , so sterilization filtration is an important link in the entire process to ensure the sterility of the liquid. , often use sterilization filtration method + aseptic production process to ensure compliance with the requirements of sterile preparations. In general eye drop production companies, sterilization is mainly for their production equipment, inner packaging materials, aseptic operating tools and other items. Yes, the medicinal solution itself is completed through sterilization and filtration.
large flow filter cartridge
The eyes are the most important sensory organ of the human body. As eye drops that act directly on the eyes, their sterility requirements are important quality indicators related to drug quality and ensuring patient safety.
Latest News
Read more
- Industry Application
- Life Sciences
- water treatment
- Industrial Filtration
- Food & Beverage
- Microelectronics
- Laboratory
- New energy battery
- Contact Us
- [email protected]
- +86-571-87022016
- +86-571-87293027
- +8613675899519
- Subscribe for Join Us!
- Join us and get detail information,technical parameter and new products etc.
CopyRight © Hangzhou Eternalwater Filtration Equipment Co., Ltd. 2002-2025
- [email protected]
- Jenny wu
- +8613675899519
- +86(571) 87022016

 EN
EN  ES
ES AR
AR JP
JP CN
CN




