
AVL Center
2025-03-13 EternalwaterAVL Introduce
Provide bacterial challenge test, extractables test, leachables test, compatibility test, product-wet integrity parameters
and absorption validation for drug manufacturers, and conduct internal validation of the company products, providing
experimental data for validation guide. The laboratory complies with the I S O 9 0 0 1 quality management system and
adheres to the concept of data reliability to ensure the authenticity, accuracy, and traceability of data.
The main staff of the validation laboratory have rich experience in process validation, and the validation methods are
scientific and reasonable, ensuring that the filtration process validation services provided meet the requirements of global regulations. Based on the quality principle of meeting customer needs, the laboratory provides users with comprehensive validation services, including regulatory interpretation, process and validation report evaluation and other consulting services, as well as process validation services for our company and other companies.
The laboratory is equipped with advanced validation equipment including UV visible spectrophotometer, high-efficiency liquid chromatograph, in-line sterilization system, particle counter, etc., which can provide reliable validation data for users.
AVL Project

Bacterial Challenge Test
Extractables Test
Leachables Test
Chemical Compatibility Test
Product-Wet Integrity Test
Safety Assessment
Adsorption Test
Development and Validation of Filtration Process
Overview of Sterile Filtration Validation
Performance Confirmation
Generally completed by the filter manufacturer
Microbial retention test, integrity test, biosafety test (toxicity test and endotoxin test), flow rate test,hydrostatic test, multiple sterilization test、extractable test, particle release test and fiber shedding test, etc.
Filtration Process Validation
Completed by the user of the filter or commissioned testing and inspection agency
Validation of the specific media to be filtered, combined with specific process conditions generally includes bacterial retention tests, chemical compatibility tests, extractables and leachables tests, safety assessment and adsorption assessment, etc. The operating parameters and allowable extremes in the actual production process have been covered during validation.
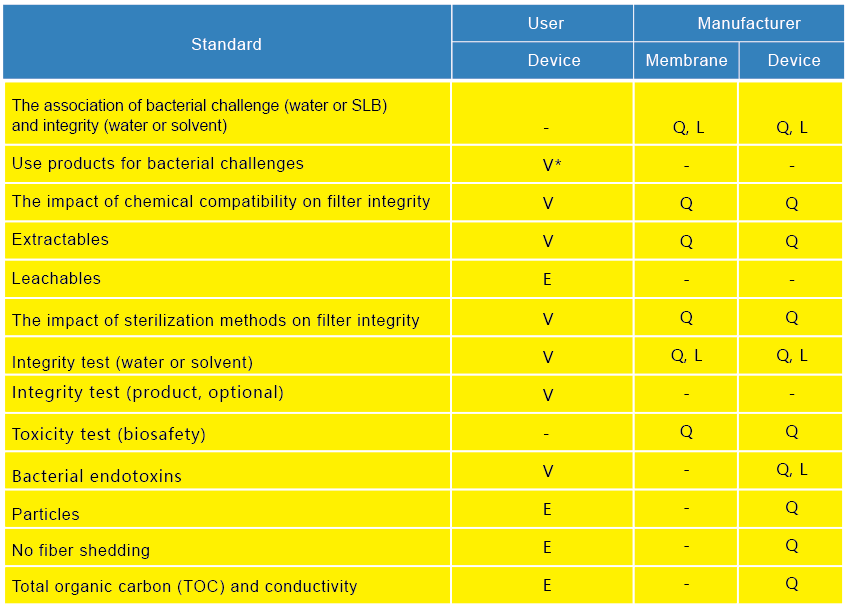
L = Batch release criteria Q = Confirmation V = Specific process validation
V*= Validate with membrane or filter E = Evaluate whether test is required
BCT
According to the test method of ASTM F838, under the specified process conditions, the challenge level ofB. diminuta ( Brevundimonas diminuta,ATCC®19146TM) in the bacterial challenge bulk is not less than 1X10 ⁷cfu/cm²

Bacterial Challenge Test (BCT) can be used to confirm whether the filter is a sterilizing-grade filter

Basic Process of BCT
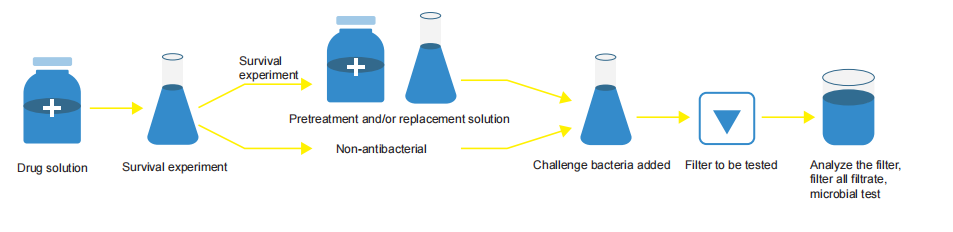

The membrane in the microbial retention test must be same as the filter material used in actual production, and should include multiple batches (usually three batches). At least one of the batches should be the low bubble point (lowspecification) filter membrane. The size of challenge microorganisms needs to be able to penetrate the 0.45
microns filter membrane to prove that it is cultivated to the appropriate size and concentration.
Extractables and Leachables Test
Under the worst process conditions, such as extreme temperature, time, etc, the data of dissolved mattersextracted after determining specific process fluids, or simulating the full contact between volatile reagents
and the membrane or filter, shall be used as the basis for drug risk assessment.

Based on the risk assessment of the potential impact of leachables on drug quality, the extractables test plan with certain "applicability" and "practicality" has been developed.
Pattern Diagram of Extractables Test
Leachables Test
Refers to compounds that migrate from any product contact material under actual process conditions. Leachables exist in the final product and are usually the subset of extractables.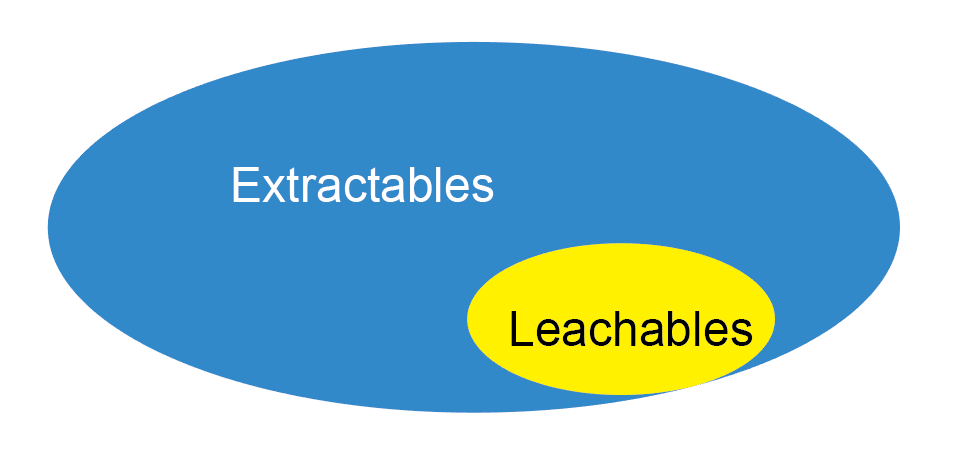
Chemical Compatibility Test
Different operating conditions and process flows may have different degrees of impact on the membrane or filter used.By measuring the physical and chemical stability of the membrane or filter, such as bubble point, membrane thickness, flow rate and other parameter changes, the compatibility of the membrane or filter with the specific process fluid can
be accurately evaluated
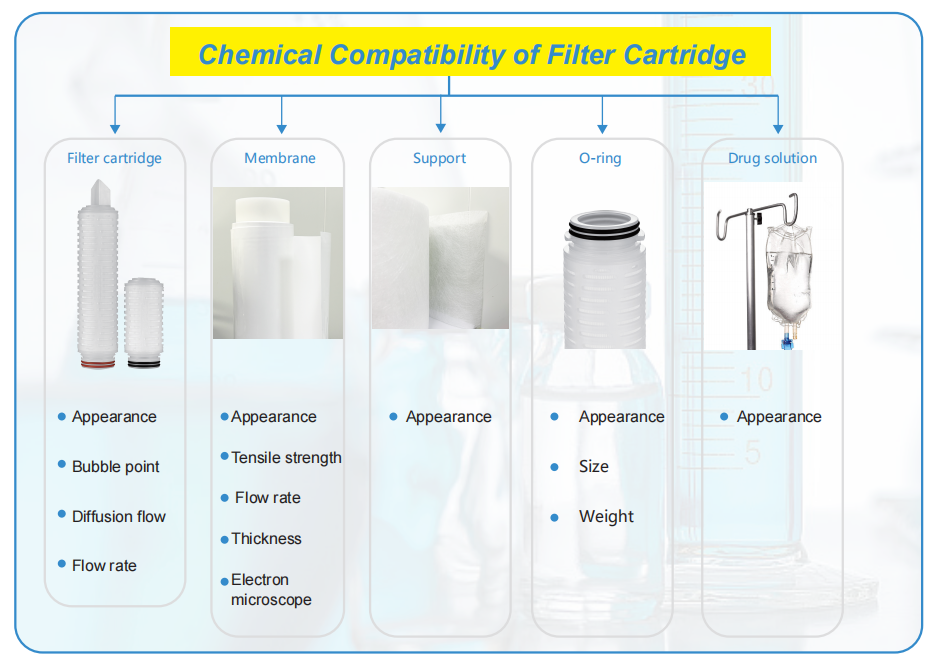
“When considering chemical compatibility, it is important to include all the components of the filter. . . ”
“Integrity testing is a physical test method associated with bacterial challenge test, and it is a method of compatibility test.”
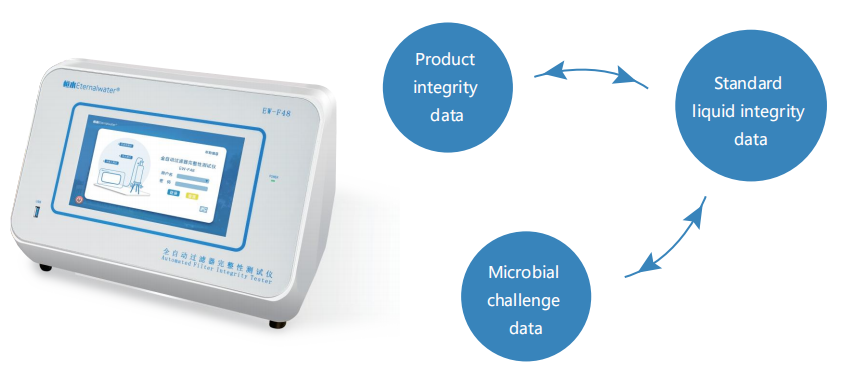
Product-Wet Integrity Test
Wet the membrane or filter with a specific process fluid and measure the bubble point, diffusion flow or pressure holding limit of the filter wetted with the specific product at a specified temperature.
Advantages of Product Wetting:
1、The integrity can be tested after filtration without complicated cleaning steps, and the integrity is easier to pass2、The disposable system can also be tested with product integrity standard before use The hydrophobic filter
cartridge should not be introduced to the non-production of homologous organic reagents and the hydrophilic
filter cartridge should avoid the impact of residual moisture on the concentration of the drug solution.
3、The cleaning of the disposable system after use requires a higher capacity of the waste liquid bag. The cleaning
step can be omitted after the validation is completed.
Only by establishing the association between integrity and microbial challenges, can the sterilization performance of
the filter cartridge be monitored by monitoring their integrity, and the integrity test is meaningful.
Development and Validation of Filtration Process
For new drug solutions, filtration process development can meet the needs of optimizing the best filtration solution(load capacity, time, filtration efficiency, etc.) under specific process conditions.
- Industry Application
- Life Sciences
- water treatment
- Industrial Filtration
- Food & Beverage
- Microelectronics
- Laboratory
- New energy battery
- Contact Us
- [email protected]
- +86-571-87022016
- +86-571-87293027
- +8613675899519
- Subscribe for Join Us!
- Join us and get detail information,technical parameter and new products etc.
- [email protected]
- Jenny wu
- +8613675899519
- +86(571) 87022016

 EN
EN  ES
ES AR
AR JP
JP CN
CN




